
SO2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
This widget gets the Lewis structure of chemical compounds. Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
SO2(Sulfur Dioxide) Molecular Geometry & Lewis Structure Geometry of Molecules
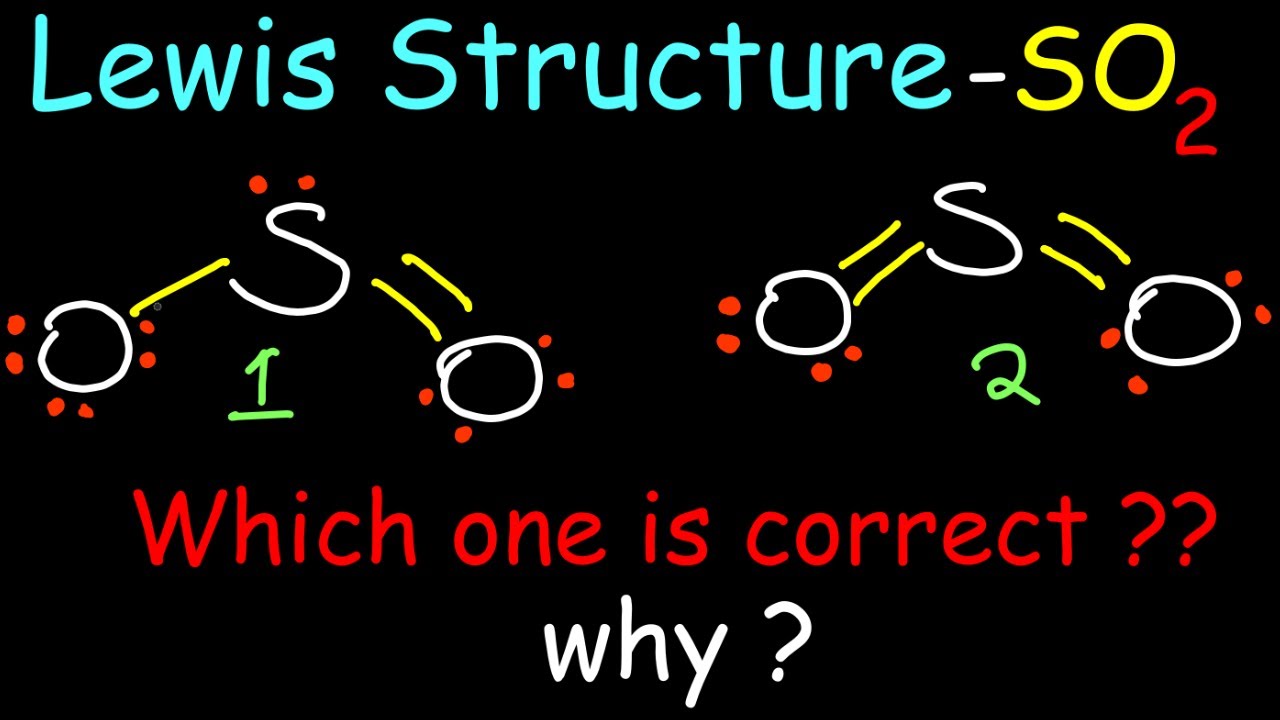
Drawing the Lewis Structure for SO 2. The Lewis structure for SO 2 requires you to place more than 8 valence electrons on Sulfur (S). You might think you've got the correct Lewis structure for SO 2 at first. Remember, Sulfur is in Period 3 and can hold more than 8 valence electrons. You'll want to calculate the formal charges on each atom to.
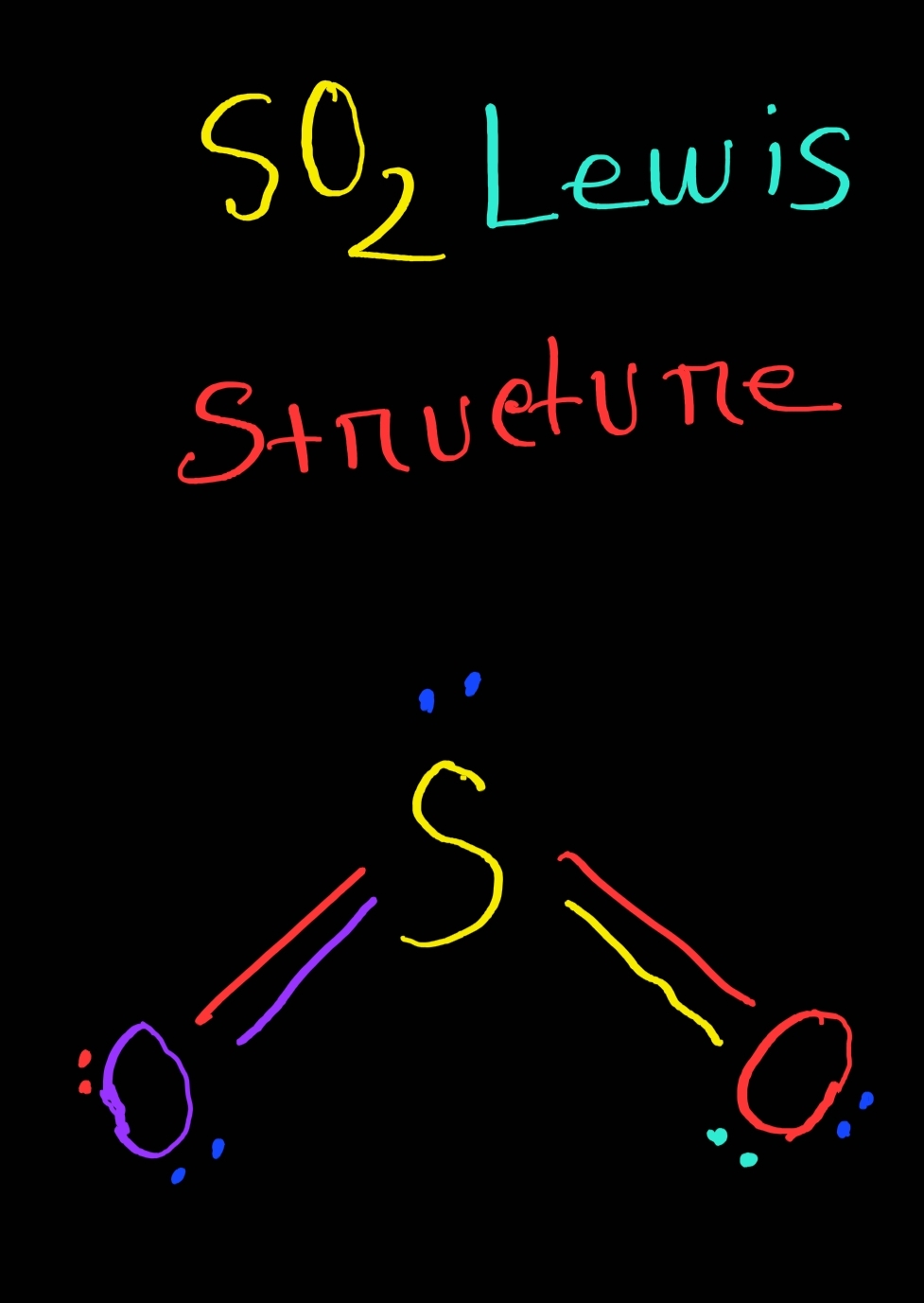
SO2 Lewis Structure ,Valence Electrons ,Formal Charge Lewis Structure for SO2 (Sulfur Dioxide)
This chemistry video tutorial explains how to draw the lewis structure of SO2 also known as Sulfur Dioxide. It discusses the molecular geometry, bond angle, hybridization and formal charges.
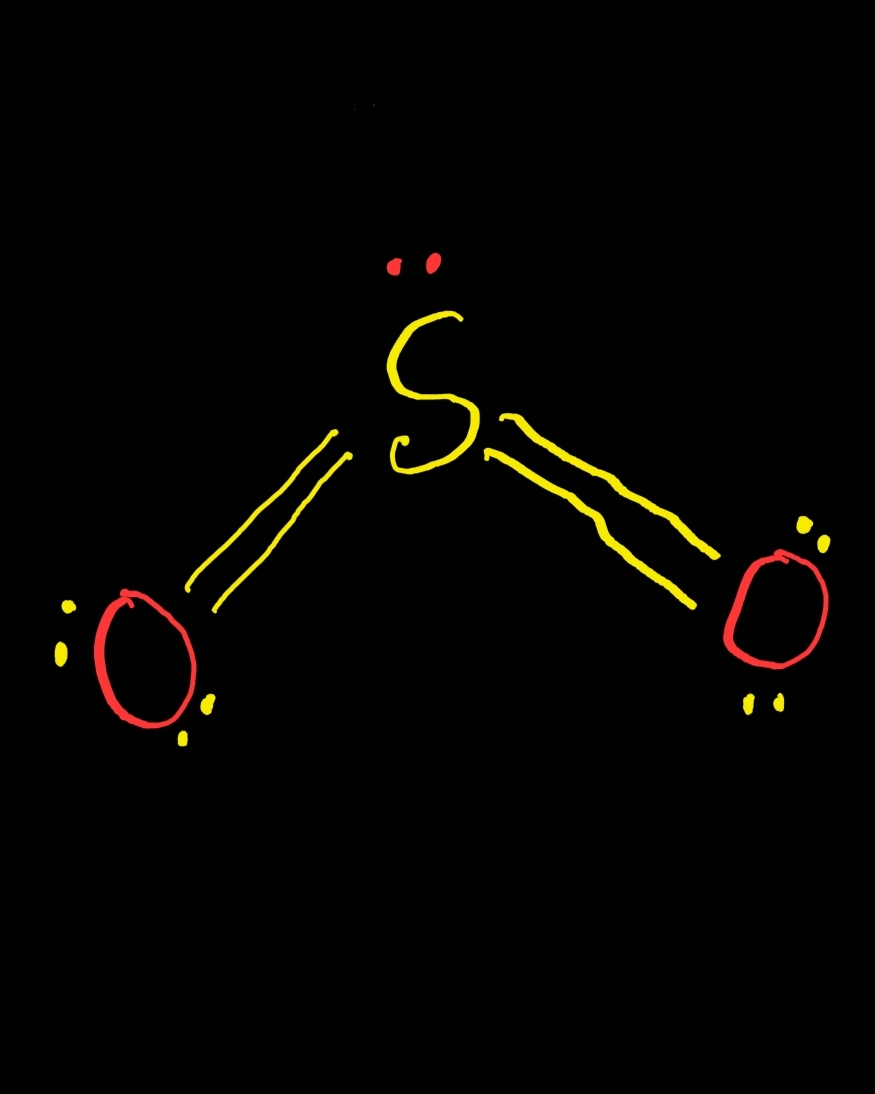
SO2 Lewis Structure ,Valence Electrons ,Formal Charge Lewis Structure for SO2 (Sulfur Dioxide)
A dot structure is any representation of atoms/molecules using dots for electrons. And a Lewis diagram (or Lewis structure or Lewis dot structure) is a type of dot structure created by the chemist Gilbert N. Lewis which is most commonly used in chemistry nowadays. There's a slight difference, but they effectively mean the same thing.
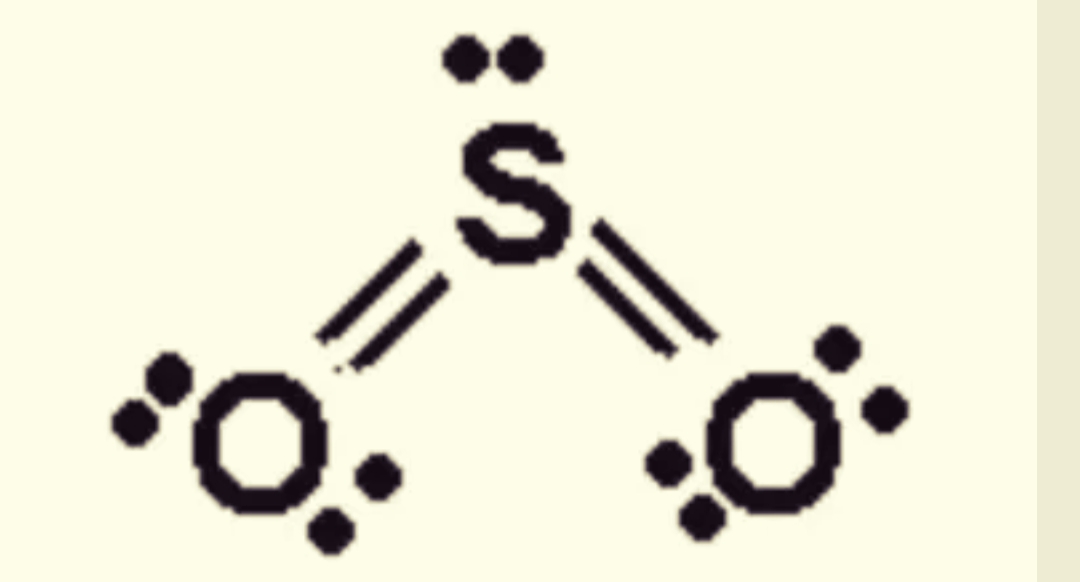
SO2 Lewis Structure ,Valence Electrons ,Formal Charge Lewis Structure for SO2 (Sulfur Dioxide)
Exercise Draw Lewis dot structures for CH 4, NH 3, HF, OF 2, F 2, O 2, N 2, Cl − and some compounds you know. Formal Charge The formal charge on any atom in a Lewis structure is a number assigned to it according to the number of valence electrons of the atom and the number of electrons around it.
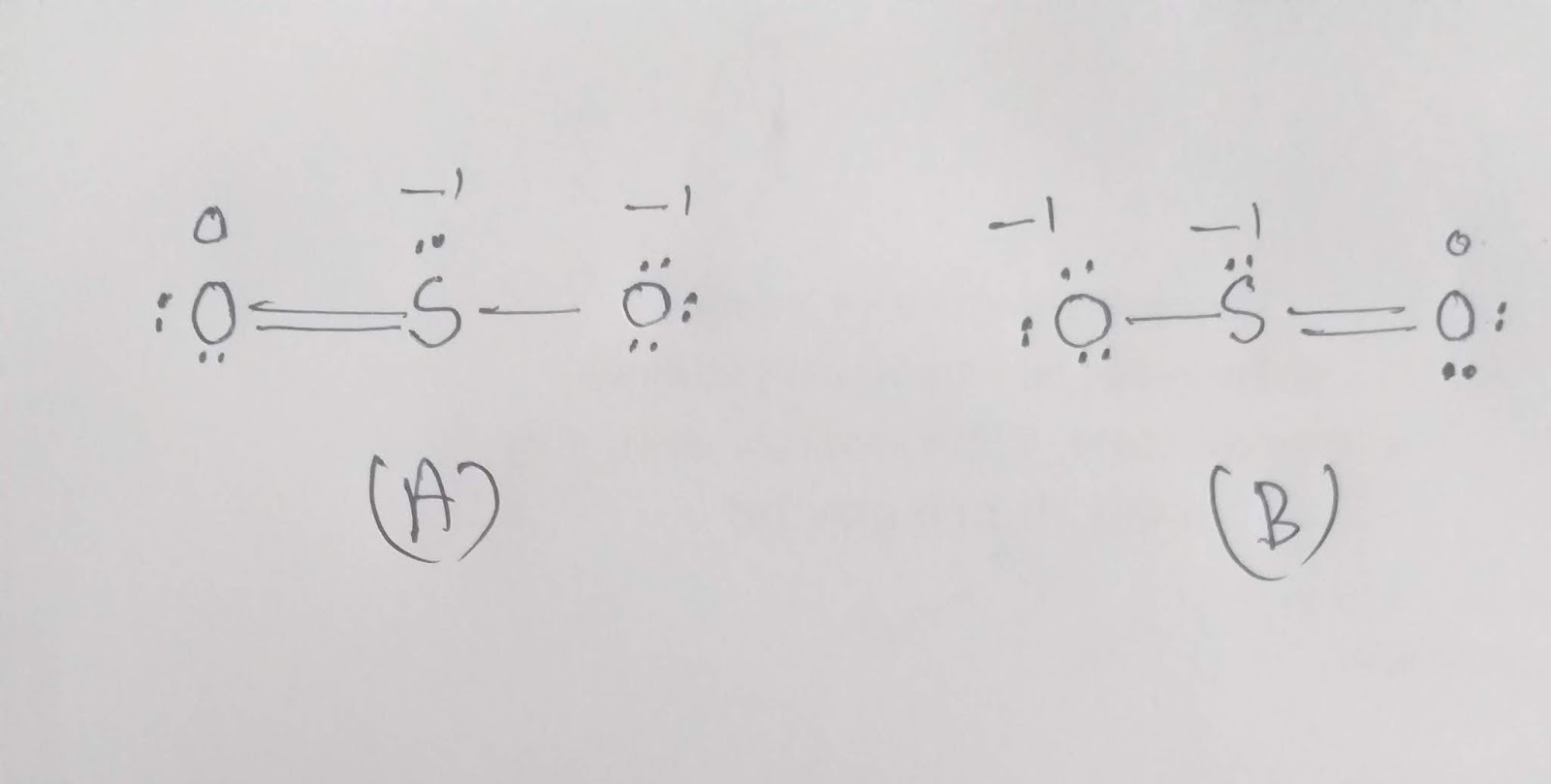
SO2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
This video shows how to draw Lewis dot structure of SO2 - how to draw a coordinated bond by using Chemsketch

How to draw SO2 Lewis Structure? Science Education and Tutorials
Lewis Structure of SO2 (sulfur dioxide) - YouTube 0:00 / 4:59 Lewis Structure of SO2 (sulfur dioxide) chemistNATE 259K subscribers Subscribe 6.6K Share 902K views 9 years ago Lewis.

SO2 Lewis Structure ,Valence Electrons ,Formal Charge Lewis Structure for SO2 (Sulfur Dioxide)
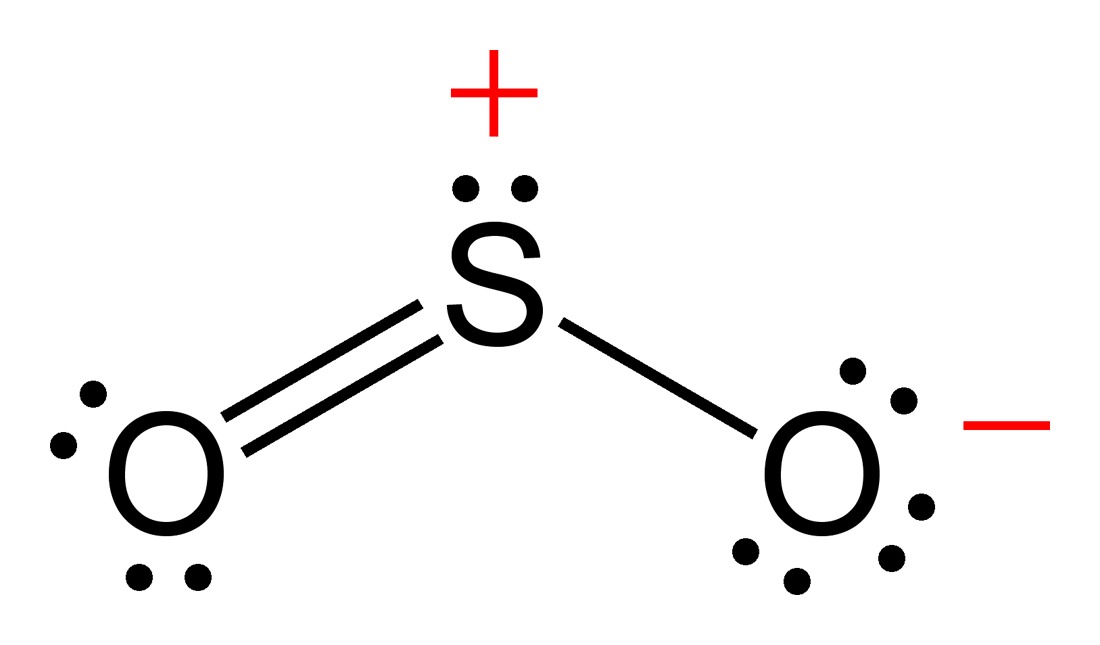
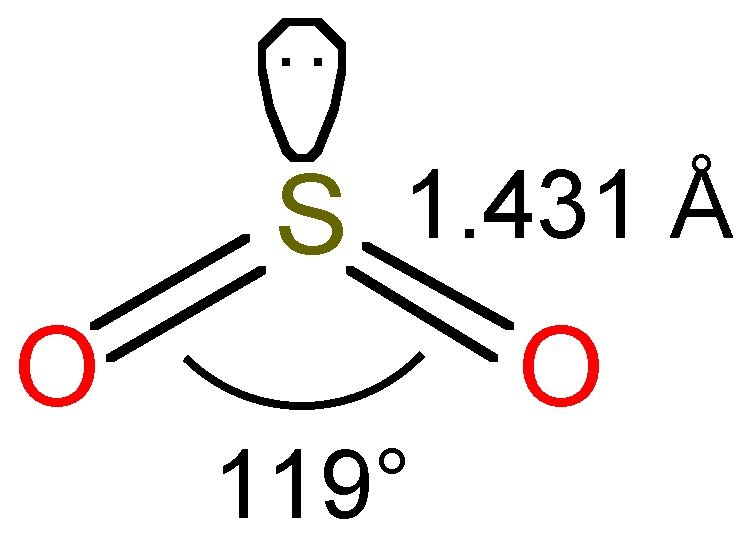
Lewis structure of SO 2 There are two double bonds between sulfur atom and oxygen atoms in SO molecule. Also, a lone pair exists on sulfur atom and each oxygen atom has two lone pairs in SO 2 lewis structure. Hybridization of SO 2 All atoms have sp 2 hybridization. Each oxygen atom has one sigma bond and two lone pairs.
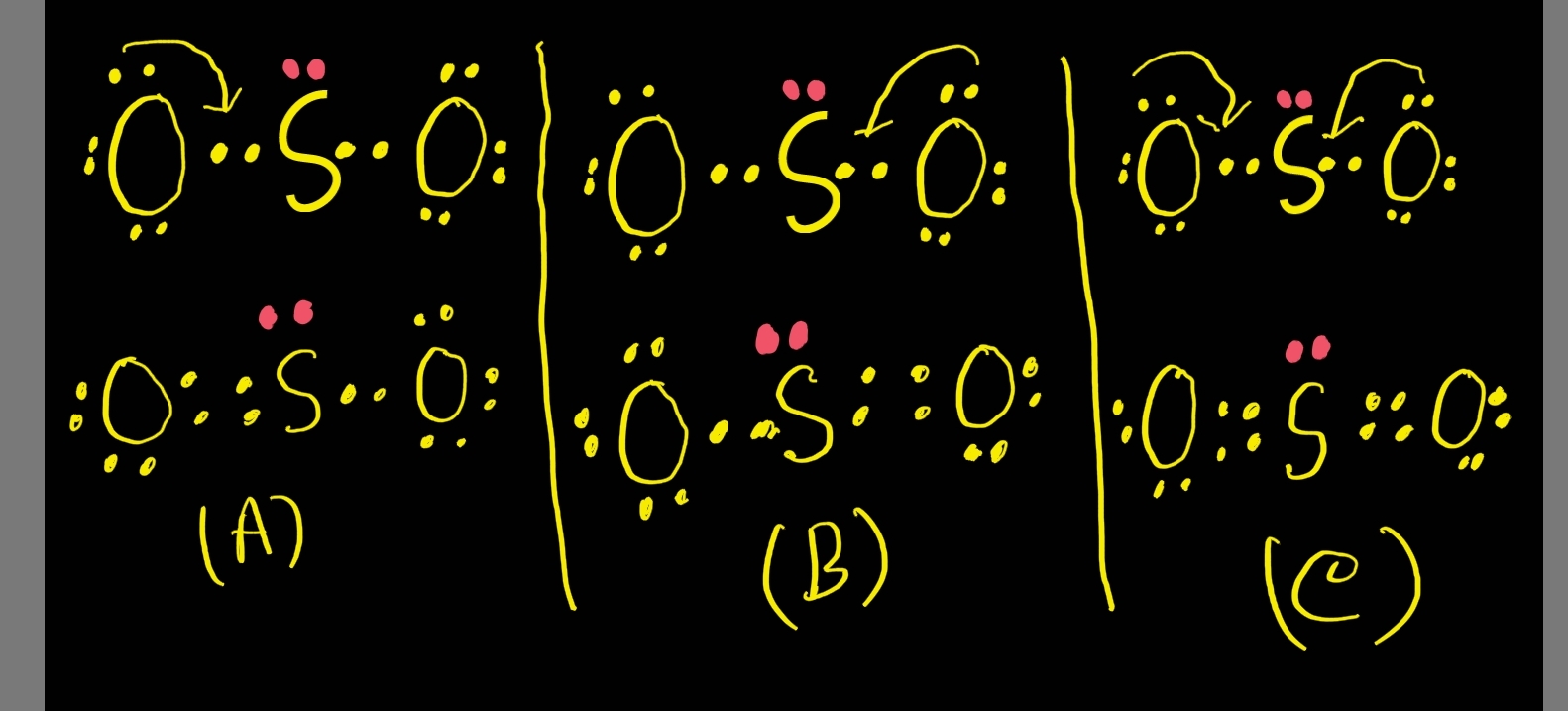
SO2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".
SO2(Sulfur Dioxide) Molecular Geometry & Lewis Structure Geometry of Molecules
Lewis structure is the distribution of the electrons around the atoms of a compound. This structure helps us to know about the kind of bonds and the number of bonds that form the compound. Now let's walk through the method of drawing lewis structure:

SO2 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagram Techiescientist
Sulfor dioxide: Lewis dot structure for SO2 (video) | Khan Academy Chemistry library Course: Chemistry library > Unit 9 Lesson 4: Dot structures and molecular geometry Resonance and dot structures Formal charge Formal charge and dot structures Worked example: Using formal charges to evaluate nonequivalent resonance structures

SO2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
A step-by-step explanation of how to draw the SO2 Lewis Structure (Sulfur Dioxide) Note: From an experimental view (using x-ray crystallography or someth.
Lewis Structure of Sulphur Dioxide SO2 YouTube
Subtract step 1 total from step 2. 24-18=6e-. Step 4: Find number of bonds by diving the number in step 3 by 2 (because each bond is made of 2 e-) 6e-/2= 3 bond pairs. Step 5: Find the number of nonbonding (lone pairs) e-. Subtract step 3 number from step 1. 18-6= 12e-=6 lone pairs. Now, use the information from step 4 and 5 to draw the Lewis.

SO2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
To form the Lewis structure of Sulfur Dioxide, we need first to determine the number of valence electrons available. These valence electrons act as the building blocks of the structure. They are found in the atom's outermost shell, where the force of attraction from the nucleus is the weakest.
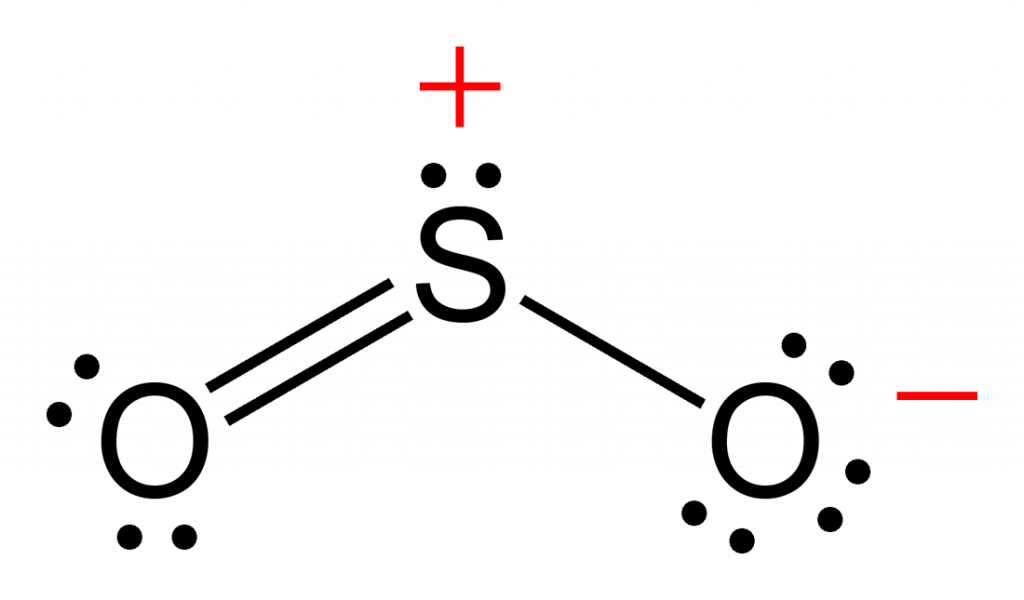
SO2(Sulfur Dioxide) Molecular Geometry & Lewis Structure Geometry of Molecules
To draw the SO2 Lewis structure, follow these simple steps: 1. Determine the total valence electrons Start by counting the valence electrons of each atom in the molecule. In SO2, sulfur is in Group 6, so it has 6 valence electrons, while each oxygen atom in Group 6 contributes 6 valence electrons.

How to draw SO2 Lewis Structure? Science Education and Tutorials
A Lewis structure is a way to show the shape of a molecule. Dots show where electrons are around the atoms, and lines or pairs of dots show where covalent bonds connect the atoms. By drawing a Lewis dot structure, you can find the lone electron pairs in molecules, which helps you figure out how chemical bonds form.